Root Causes of Heart Disease, Cancer & Diabetes
Explore the common driver behind heart disease, cancer, diabetes, and other diseases. Learn about the root-cause triad and two natural compounds that address oxidative stress, inflammation, and insulin resistance, backed by 38 studies.
FATTY LIVER DISEASECANCERCARDIOVASCULAR HEALTHMETABOLIC HEALTHTYPE 2 DIABETESBRAIN HEALTHINFLAMMATIONBONE HEALTHMUSCLE HEALTHCELLULAR HEALTH
Natural Health Connect Research Team
1/16/202613 min read
The Problem With Modern Healthcare
Your doctor prescribes statins for high cholesterol. Another specialist adds metformin for prediabetes. A third recommends bisphosphonates for thinning bones. Each medication targets a different symptom, yet they're all downstream consequences of the same underlying dysfunction.
What if we told you that heart disease, type 2 diabetes, cancer, dementia, osteoporosis and accelerated ageing all share three common root causes? And that addressing these root causes, rather than treating individual symptoms, could prevent or reverse multiple chronic diseases simultaneously?
This isn't wishful thinking. It's backed by hundreds of peer-reviewed studies, recent clinical trials, and emerging research that's rewriting our understanding of chronic disease.
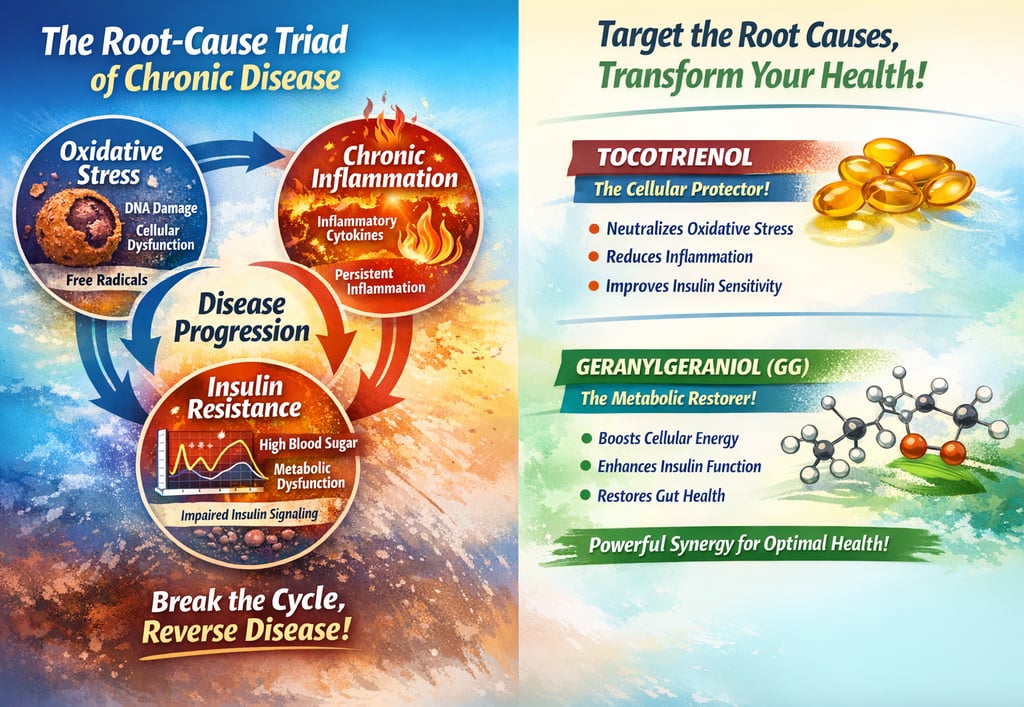
The Root-Cause Triad - Three Interconnected Mechanisms Driving Nearly Every Chronic Disease
Modern research has identified three cellular mechanisms that fuel the development and progression of most chronic diseases:
1. Oxidative Stress
Excess free radicals (reactive oxygen species, or ROS) damage DNA, proteins and mitochondria, accelerating cellular ageing and disease progression. Think of it as cellular rust that accumulates faster than your body can clean it up.
2. Chronic Inflammation
Persistent activation of inflammatory pathways (particularly NF-κB) releases pro-inflammatory cytokines like IL-6, TNF-α, and CRP. Unlike acute inflammation (which heals injuries), chronic inflammation creates a smoldering fire that fuels metabolic dysfunction, neurodegeneration and cancer growth.
3. Insulin Resistance
Impaired insulin signalling (via PI3K/Akt/mTOR pathways) drives hyperinsulinemia (elevated insulin levels), hyperglycemia (high blood sugar), and elevated IGF-1, a potent growth factor that accelerates both ageing and cancer cell proliferation.
Here's the critical insight: These three mechanisms don't operate independently. They create a self-perpetuating cycle:
Oxidative stress triggers inflammation
Inflammation worsens insulin resistance
Insulin resistance generates more oxidative stress and inflammation
The cycle accelerates, driving disease progression
The Insulin Resistance-Cancer Connection, A Hidden Epidemic
One of the most startling discoveries in recent research is the direct link between insulin resistance and cancer risk.
A 2025 longitudinal study tracking over 100,000 participants found that chronic inflammation and insulin resistance synergistically increase cancer risk by 71% when both are elevated. The study revealed that inflammation appears to be the primary driver of future insulin resistance changes, creating a cascade that promotes cancer initiation and progression.
How Does This Happen?
When you develop insulin resistance, your pancreas compensates by producing more insulin (hyperinsulinemia). This excess insulin doesn't just affect blood sugar, it directly activates cancer growth pathways:
The PI3K/Akt Pathway Activation
Hyperinsulinemia activates PI3K/Akt signalling in cells throughout the body
This same pathway is one of the most commonly mutated in cancers
Activated PI3K/Akt promotes:
Glucose uptake (feeding cancer cells)
Cell survival (preventing cancer cell death)
Angiogenesis (building blood vessels to tumours)
Metastasis (cancer spread)
IGF-1 Elevation
High insulin increases bioavailable IGF-1, a potent tumour growth factor
IGF-1 binds to receptors on cancer cells, triggering the same PI3K/Akt cascade
This creates a "perfect storm" for tumour development and progression
Chronic Inflammatory Environment
Insulin resistance triggers persistent inflammation
Elevated IL-6, TNF-α, and NF-κB create a tumour-promoting microenvironment
This inflammation suppresses immune surveillance, allowing cancer cells to evade detection
The evidence is stark
Insulin-resistant individuals have significantly higher risk of breast, colorectal, liver, pancreatic, lung and prostate cancers.
Why Conventional Medications Fall Short (And Sometimes Make Things Worse)
The fundamental problem with most prescription drugs is that they treat symptoms, not root causes. Worse, some medications actually worsen the underlying triad:
Statins
While lowering cholesterol, statins deplete both CoQ10 and geranylgeranyl pyrophosphate (GGPP), critical compounds for cellular energy production and insulin signalling. This can paradoxically worsen insulin sensitivity and cause muscle damage in 10-25% of patients.
Diabetes Drugs
Most diabetes medications lower blood glucose without addressing the chronic inflammation driving insulin resistance. The underlying metabolic dysfunction continues unchecked.
Cancer Chemotherapy
While killing cancer cells, chemotherapy generates massive oxidative stress and cellular damage. Without metabolic protection, this increases recurrence risk by failing to address the metabolic environment that allowed cancer to develop initially.
Bisphosphonates (Osteoporosis Drugs)
These drugs prevent bone breakdown but don't address why bone formation has slowed. Some research suggests they may actually impair the bone-building process over time.
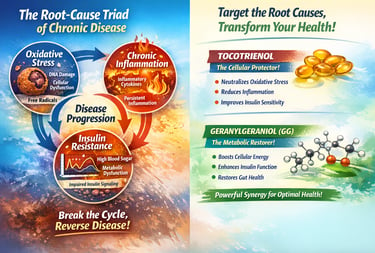
The Solution - Two Natural Compounds That Address All Three Pillars Simultaneously
Emerging research has identified two compounds naturally extracted from annatto seeds that uniquely target the complete root-cause triad:
TOCOTRIENOL - The Cellular Protector
Tocotrienols are the lesser-known but far more potent forms of vitamin E, unlike alpha-tocopherol (the common vitamin E supplement). Delta- and gamma-tocotrienol isomers are:
40-60× more potent antioxidants than the common vitamin E
Able to cross the blood-brain barrier (rare for nutrients)
Direct inhibitors of inflammatory and cancer-promoting pathways
How Tocotrienol Addresses the Triad
1. Neutralizes Oxidative Stress
Penetrates cell membranes more efficiently due to unsaturated side chain
Scavenges free radicals at the cellular level
Upregulates endogenous antioxidant enzymes (SOD, catalase)
2. Suppresses Chronic Inflammation
Inhibits NF-κB activation, the master switch for inflammatory gene expression
Reduces pro-inflammatory cytokines (IL-6, TNF-α, CRP) by 10-20% in clinical trials
Downregulates inflammatory signalling in multiple tissues
3. Improves Insulin Sensitivity
Activates PPARγ/α/δ, nuclear receptors that enhance insulin signalling
Protects IRS-1 (insulin receptor substrate) from oxidative damage, restoring cellular insulin response
Reduces adipose tissue inflammation, a key driver of insulin resistance
Tocotrienol's Unique Anti-Cancer Mechanisms
A groundbreaking 2023 Phase II clinical trial tested delta-tocotrienol combined with standard chemotherapy in 60 breast cancer patients. The study demonstrated:
Safety and feasibility when combined with neoadjuvant chemotherapy
Synergistic anticancer effects without increasing side effects
Potential to enhance treatment response
The mechanisms behind this are remarkable:
Direct Pathway Inhibition
Blocks NF-κB and PI3K/Akt. the exact pathways hyperinsulinemia activates in cancer cells
Induces apoptosis (cancer cell death) via caspase activation and TRAIL upregulation
Reverses epithelial-mesenchymal transition (EMT), preventing metastasis
Cancer Stem Cell Targeting
Works at the cancer stem cell level, the root of tumor recurrence
Suppresses self-renewal pathways that allow cancer to regenerate after treatment
Enhances chemotherapy effectiveness by overcoming drug resistance
Anti-Angiogenesis
Suppresses VEGF (vascular endothelial growth factor)
Cuts off blood supply to tumours, starving cancer cells
Clinical Evidence:
Victoria University meta-analysis (300+ studies, 2022)
Pakistan RCTs showing HbA1c reduction and NAFLD improvement
Ongoing trials: NCT06519097 (pancreatic cyst prevention), NCT02909751 (advanced cancer adjuvant therapy)
Neuroprotection Beyond Compare
Tocotrienols are one of the few nutrients capable of crossing the blood-brain barrier, a critical advantage for preventing neurodegenerative diseases.
Research shows tocotrienols:
Reduce amyloid-β plaques in Alzheimer's models
Protect dopaminergic neurons in Parkinson's disease
Suppress neuroinflammation via NF-κB inhibition
Restore cognitive function in animal models of neurodegeneration
Bone Building Without Side Effects
Unlike bisphosphonates (which only prevent bone breakdown), tocotrienols actually stimulate bone formation while suppressing bone resorption:
Enhance osteoblast (bone-building cell) differentiation and activity
Suppress osteoclast (bone-breakdown cell) formation via RANKL/OPG regulation
Improve bone mineral density and microarchitecture in animal models
Work through mevalonate pathway modulation and anti-inflammatory effects
GERANYLGERANIOL (GG) - The Metabolic Restorer
Geranylgeraniol is a mevalonate pathway intermediate that your body converts to geranylgeranyl pyrophosphate (GGPP), a critical molecule for both energy production and cellular signalling.
How Geranylgeraniol Addresses the Triad
1. Restores Cellular Energy (Mitochondrial Function)
Enables endogenous CoQ10 synthesis, your mitochondria produce their own CoQ10 rather than relying on poorly absorbed external supplements
CoQ10 is the electron transport chain catalyst essential for ATP (cellular energy) production
Reverses mitochondrial dysfunction caused by oxidative stress and ageing
2. Directly Improves Insulin Sensitivity
Activates PPARγ, the master regulator of insulin sensitivity and glucose metabolism
Enables protein prenylation via GGPP, which is essential for:
Proper insulin receptor function
GLUT4 glucose transporter translocation to cell membranes
Small GTPase function (Ras, Rho, Rac) critical for insulin signajling
3. Modulates Inflammation via Multiple Pathways
Gut microbiome rebalancing: Increases anti-inflammatory bacterial species (Butyricicoccus) while reducing obesity-associated species
Reduces pro-inflammatory adipokines (leptin, MCP-1, TNF-α) from fat tissue
Addresses metabolic endotoxemia, chronic low-grade inflammation from gut dysbiosis
Clinical Evidence - Reversing Metabolic Dysfunction
Texas Tech & University of Missouri Studies (2021)
In high-fat diet-induced obese mice, geranylgeraniol supplementation:
Improved glucose tolerance by 35-40%
Enhanced insulin sensitivity significantly
Reduced pro-inflammatory adipokines across multiple markers
Increased bone formation markers while decreasing bone resorption markers
Improved bone microstructure: increased femur stiffness and cortical thickness
The mechanism? PPARγ activation combined with gut microbiome modulation created a comprehensive metabolic reset.
The Statin Muscle Damage Solution - A Clinical Breakthrough
For years, statin-induced muscle pain (affecting 10-25% of patients) was considered an unavoidable trade-off. Many patients stopped taking statins, losing the intended cardiovascular protection.
The science was clear, statins deplete GGPP, leading to impaired protein prenylation in muscle cells. This triggers atrogin-1 expression, a marker of muscle atrophy, causing pain, weakness and fatigue.
The Breakthrough
Multiple studies have now demonstrated that geranylgeraniol supplementation:
Completely reverses statin-induced muscle damage in animal models
Prevents force production decline in fast-twitch muscle fibers
Suppresses atrogin-1 expression by 65%, reversing the muscle atrophy signal
Restores myotube and myofiber morphology to normal
Does NOT interfere with statin's cholesterol-lowering effect
Human Evidence:
In ex vivo human muscle samples, statins increased atrogin-1 by over 400%. This massive spike indicates severe muscle damage, explaining the debilitating pain patients experience.
Clinical Implication: Geranylgeraniol allows patients to stay on statin medications without suffering muscle damage. This is a genuine clinical breakthrough that addresses one of the most common reasons for statin discontinuation.
Why Together? The Synergistic Effect
Tocotrienol and geranylgeraniol don't just work independently, they create a synergistic intervention that addresses all three pillars of the root-cause triad more comprehensively than either could alone.
The Synergy in Action
Pillar 1: Oxidative Stress
Tocotrienol: Neutralises ROS, restores IRS-1 insulin signalling proteins
Geranylgeraniol: Enables endogenous antioxidant enzyme production
Combined Result: Complete ROS neutralisation at both acute and chronic levels
Pillar 2: Chronic Inflammation
Tocotrienol: Inhibits NF-κB, reduces inflammatory cytokine production
Geranylgeraniol: PPARγ activation, gut microbiome rebalancing reduces systemic inflammation
Combined Result: Multi-site anti-inflammatory action (cellular + metabolic + microbiome)
Pillar 3: Insulin Resistance
Tocotrienol: PPARγ/α/δ activation, direct IRS-1 protection from oxidative damage
Geranylgeraniol: GGPP-mediated insulin receptor function, GLUT4 translocation
Combined Result: Comprehensive insulin sensitivity restoration at receptor, signalling and metabolic levels
Additional Synergies
Energy Restoration
Tocotrienol: Supports mitochondrial membrane integrity and function
Geranylgeraniol: Enables CoQ10 synthesis for electron transport chain
Combined Result: Full ATP/energy recovery in insulin-resistant and ageing cells
Cancer Prevention & Adjuvant Support
Tocotrienol: Blocks cancer growth pathways (apoptosis, EMT reversal, angiogenesis suppression)
Geranylgeraniol: Immune activation, metabolic normalisation (reduces hyperinsulinemia)
Combined Result: Prevention addresses metabolic drivers + adjuvant enhances treatment effectiveness
This is why Wellness Extract, our manufacturing partner, formulated them together, addressing the complete root-cause triad which requires both lipophilic oxidative stress reduction (tocotrienol) AND metabolic/mitochondrial restoration (geranylgeraniol).
Real-World Applications - Who Benefits?
Cardiovascular Disease Prevention & Reversal
The most compelling evidence comes from carotid artery disease studies: 88% of patients showed regression (reversal) of arterial plaque with 240mg/day tocotrienol supplementation. Combined with geranylgeraniol's insulin sensitivity improvements and CoQ10 restoration, this creates comprehensive cardiovascular protection.
For statin users: Add geranylgeraniol to prevent muscle damage while maintaining cholesterol-lowering benefits.
Type 2 Diabetes & Metabolic Syndrome
Research shows tocotrienol performs equivalently to metformin in glucose control studies. But unlike metformin, it also:
Builds bone density (metformin doesn't)
Reduces chronic inflammation
Crosses the blood-brain barrier for neuroprotection
Combined with geranylgeraniol's PPARγ activation and microbiome modulation, this addresses the root metabolic dysfunction, not just the symptom of high blood sugar.
Cancer Prevention (Metabolic Risk Factors)
If you have obesity, insulin resistance, pre-diabetes, or family history of cancer, you face elevated cancer risk due to the hyperinsulinemia-inflammation-oxidative stress triad.
The intervention: Tocotrienol + geranylgeraniol address the root metabolic drivers of cancer initiation by:
Lowering hyperinsulinemia (via insulin sensitivity restoration)
Suppressing chronic inflammation (NF-κB inhibition + microbiome modulation)
Neutralising oxidative stress and DNA damage
Blocking PI3K/Akt pathway activation
Cancer Adjuvant Therapy
For patients undergoing chemotherapy, tocotrienol has demonstrated:
Enhanced treatment response (amplifies chemotherapy effectiveness)
Reduced drug resistance in cancer cells
Cancer stem cell targeting (prevents recurrence)
Metabolic protection during treatment (reduces oxidative damage to healthy cells)
Combined with geranylgeraniol for immune function support and metabolic normalisation, this creates a comprehensive adjuvant strategy.
Dementia & Cognitive Decline Prevention
With the brain using 20% of the body's energy, mitochondrial support is critical. Tocotrienol's ability to cross the blood-brain barrier provides:
Direct neuroprotection against oxidative stress
Reduced amyloid-β and tau protein aggregation
Suppressed neuroinflammation
Improved cerebral blood flow
Geranylgeraniol restores brain cell energy production through CoQ10 synthesis, supporting the high metabolic demands of neural tissue.
Osteoporosis Prevention & Reversal
Unlike bisphosphonates (which only prevent bone breakdown), this combination:
Stimulates osteoblast activity (bone formation)
Suppresses osteoclast formation (bone resorption)
Reverses drug-induced bone damage (geranylgeraniol restores GGPP-dependent osteoblast function)
Builds bone while improving metabolic health (no other intervention does this)
The Bigger Picture - Prevention Medicine vs. Disease Management
The conventional medical model treats diseases after they manifest. It's reactive, symptom-focused, and often requires lifelong medication with side effects.
The prevention medicine model addresses root causes before disease develops, or reverses early-stage dysfunction before it progresses.
This isn't theoretical. The research demonstrates:
Carotid artery plaque regression (not just prevention)
Bone density improvement (not just maintenance)
Insulin sensitivity restoration (not just glucose lowering)
Cancer pathway inhibition (not just tumour treatment)
Cognitive function preservation (not just symptom management)
The root-cause triad framework explains why this works
When you address oxidative stress, chronic inflammation, and insulin resistance simultaneously, you're not treating seven different diseases. You're resolving the three underlying dysfunctions that create multiple disease manifestations.
Key Takeaways
Most chronic diseases share three common root causes: oxidative stress, chronic inflammation and insulin resistance. These create a self-perpetuating cycle.
Insulin resistance + chronic inflammation = 71% increased cancer risk. The hyperinsulinemia-PI3K/Akt-IGF-1 cascade directly promotes tumour growth.
Conventional medications treat symptoms, not root causes. Some (like statins and bisphosphonates) may worsen underlying metabolic dysfunction.
Tocotrienol addresses oxidative stress + inflammation + insulin resistance through:
40-60× superior antioxidant activity vs. standard vitamin E
NF-κB and PI3K/Akt pathway inhibition
PPARγ activation and IRS-1 protection
Blood-brain barrier penetration
Cancer growth pathway suppression
Geranylgeraniol addresses insulin resistance + energy depletion through:
Endogenous CoQ10 synthesis (superior to external supplementation)
PPARγ activation and GGPP-mediated insulin receptor function
Gut microbiome rebalancing
Reversal of statin-induced muscle damage
Together, they create comprehensive root-cause intervention that prevents or reverses multiple chronic diseases simultaneously, something no pharmaceutical approach can achieve.
Clinical evidence is substantial: 300+ studies (Victoria University meta-analysis), Phase II cancer trial, multiple diabetes and cardiovascular studies, statin myopathy reversal research, bone health trials and neuroprotection studies.
The Bottom Line
Modern medicine's single-disease, single-drug approach misses the interconnected nature of chronic disease. The root-cause triad framework, oxidative stress, chronic inflammation and insulin resistance. explains why addressing these three mechanisms simultaneously can prevent or reverse multiple diseases at once.
Tocotrienol and geranylgeraniol, extracted from annatto seeds, uniquely target all three pillars. The research is clear, the mechanisms are understood and the clinical evidence continues to accumulate.
This isn't about replacing medical care, it's about addressing the metabolic dysfunction that allows disease to develop in the first place.
Prevention medicine starts with understanding the root cause. Now you do.
References
Tocotrienol & Insulin Resistance
Chong JY, Huang TC, Chueh SM, et al. (2025). γ-Tocotrienol attenuates oxidative stress and preserves mitochondrial function in inflammation-induced muscle atrophy. Redox Biology, 87:103874.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC12508907/Rafique S, Khan DA, Farhat K, Noor M, Khan MA, Sharif M. (2023). Tocopherol Vs Tocotrienol (Vitamin E) in the Management of Metabolic Syndrome. Isra Medical Journal, 15(2):74-77.
https://www.imj.com.pk/wp-content/uploads/2024/02/RA-52-07-23.pdfMechanistic Insights into Antioxidant Interventions Targeting Obesity-Induced Oxidative Stress (2025). Current Issues in Molecular Biology, 47(12):1063.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC12731757/
Geranylgeraniol & Insulin Sensitivity
Chung E, Elmassry MM, Cao JJ, et al. (2021). Beneficial effect of dietary geranylgeraniol on glucose homeostasis and bone microstructure in obese mice is associated with suppression of proinflammation and modification of gut microbiome. Nutrition Research, 93:27–37.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8464510/Matsubara T, Takakura N, Urata M, et al. (2018). Geranylgeraniol Induces PPARγ Expression and Enhances the Biological Effects of a PPARγ Agonist in Adipocyte Lineage Cells. In Vivo, 32(6):1339–1344.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6365726/Podszun MC, et al. (2021). Beneficial effect of dietary geranylgeraniol on glucose homeostasis and bone microstructure in obese mice. Nutrition & Metabolism.
https://www.sciencedirect.com/science/article/abs/pii/S0271531721000439
PI3K/Akt Pathway & Cancer
Fontana F, Giannitti G, Marchesi S, Limonta P. (2024). The PI3K/Akt Pathway and Glucose Metabolism: A Dangerous Liaison in Cancer. International Journal of Biological Sciences, 20(8):3113–3125.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11186371/Association of PTEN/PI3K/Akt pathway gene expression with insulin indices in adipose tissues (2025). Nature Scientific Reports.
https://www.nature.com/articles/s41598-025-05233-4Xu N, et al. (2018). The PI3K/AKT pathway in obesity and type 2 diabetes. International Journal of Biological Sciences, 14(11):1483–1496.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6158718/Laviola L, et al. (2020). Insulin–PI3K signalling: an evolutionarily insulated metabolic driver of cancer. Nature Reviews Endocrinology, 16:357–372.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7286536/
Tocotrienol & Cancer
Kjær IM, et al. (2023). Phase II trial of delta-tocotrienol in neoadjuvant breast cancer treatment. Nature Scientific Reports, 13:8371.
https://www.nature.com/articles/s41598-023-35362-7Ling MT, et al. (2012). Tocotrienol as a potential anticancer agent. Carcinogenesis, 33(2):233–239.
https://academic.oup.com/carcin/article/33/2/233/2463536Younes M, et al. (2024). Review: Tocotrienol isoforms—The molecular mechanisms of their anticancer effects. Gene Reports, 34:101825.
https://www.sciencedirect.com/science/article/abs/pii/S2095496424000025Naomi R, et al. (2021). An Interactive Review on the Role of Tocotrienols in the Modulation of Neurodegeneration. Frontiers in Nutrition, 8:754086.
https://www.frontiersin.org/journals/nutrition/articles/10.3389/fnut.2021.754086/full
NF-κB & Chronic Inflammation
Liu T, Zhang L, Joo D, Sun SC. (2017). NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy, 2:e17023.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5661633/Barnes PJ, Karin M. (2000). NF-κB: a key role in inflammatory diseases. Journal of Clinical Investigation, 107(1):7–11.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC198552/Yamamoto Y, Gaynor RB. (2000). NF-κB as a therapeutic target in chronic inflammation: recent advances. Trends in Pharmacological Sciences, 21(12):460–467.
https://www.sciencedirect.com/science/article/abs/pii/S0165614700018141Lawrence T. (2009). The nuclear factor NF-κB pathway in inflammation. Cold Spring Harbor Perspectives in Biology, 1(6):a001651.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2882124/Modulation of NF-κB signaling pathway by tocotrienol in neuroinflammation (2025). Antioxidants.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC12552194/
Tocotrienol Neuroprotection & Blood-Brain Barrier
Fukui K. (2019). Neuroprotective and Anti-Obesity Effects of Tocotrienols. Journal of Nutritional Science and Vitaminology, 65(Supplement):S185–S192.
https://www.jstage.jst.go.jp/article/jnsv/65/Supplement/65_S185/_pdfKumari M, et al. (2021). Tocotrienols Ameliorate Neurodegeneration and Motor Deficits in a Parkinson's Disease Model. Biomedicines, 9(5):528.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8150907/Exploring the anti-inflammatory activities, mechanism, and neuroprotective effects of tocotrienols (2023). Nutrients.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11406131/
Geranylgeraniol & Statin Muscle Damage
Tan B, et al. (2023). Potential role of geranylgeraniol in managing statin-associated muscle symptoms: a systematic review. Frontiers in Pharmacology, 14:1202485.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10691100/Irwin JC, et al. (2020). Geranylgeraniol prevents statin-induced skeletal muscle fatigue without blocking reductions in circulating cholesterol. Journal of Cachexia, Sarcopenia and Muscle, 11(5):1174–1186.
https://pubmed.ncbi.nlm.nih.gov/31491372/Jaśkiewicz A, et al. (2018). Geranylgeraniol Prevents Statin-Dependent Myotoxicity. Nutrients, 10(7):899.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5987243/Cao P, et al. (2009). Statin-induced muscle damage and atrogin-1 induction is the result of a geranylgeranylation defect. FASEB Journal, 23(11):3746–3757.
https://faseb.onlinelibrary.wiley.com/doi/full/10.1096/fj.08-128843Barajas B, et al. (2022). Geranylgeranyl pyrophosphate depletion by statins compromises skeletal muscle insulin sensitivity. Journal of Cachexia, Sarcopenia and Muscle, 13(3):1646–1660.
https://onlinelibrary.wiley.com/doi/full/10.1002/jcsm.13061
Tocotrienol & Bone Health
Chin KY, Ima-Nirwana S. (2020). Tocotrienols in Bone Protection: Evidence from Preclinical Studies. eFood, 1(1):63–77.
https://onlinelibrary.wiley.com/doi/full/10.2991/efood.k.200427.001Chin KY, Ima-Nirwana S. (2015). The biological effects of tocotrienol on bone: a review on evidence from rodent models. Drug Design, Development and Therapy, 9:2471–2482.
https://www.dovepress.com/the-biological-effects-of-tocotrienol-on-bone-a-review-on-evidence-fro-peer-reviewed-fulltext-article-DDDTWong SK, et al. (2018). Tocotrienols Regulate Bone Loss through Suppression on Osteoclast Differentiation and Activity: A Systematic Review. Current Drug Targets, 19(9):1095–1107.
https://pubmed.ncbi.nlm.nih.gov/29412105/
Insulin Resistance, Inflammation & Cancer Connection
Temporal relationship between chronic inflammation and insulin resistance and their combined cumulative effect on cancer risk: a longitudinal cohort study (2025). BMC Medicine, 23:162.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC12016061/Kim EY, et al. (2022). Prognostic importance of systemic inflammation and insulin resistance in patients with cancer: a prospective multicenter study. Nutrients, 14(12):2457.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9233357/Gallagher EJ, LeRoith D. (2011). Insulin resistance and cancer risk: an overview of the pathogenesis. American Journal of Lifestyle Medicine, 6(4):278–287.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3372318/Vigneri R, et al. (2010). The Proliferating Role of Insulin and Insulin-Like Growth Factors in Cancer. Endocrine, 39(9):899–913.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2949481/Pollak M. (2008). Insulin and insulin-like growth factor signalling in neoplasia. Nature Reviews Cancer, 8:915–928.
https://www.nature.com/articles/nrc2536
Additional Supporting Evidence
Looi AD, et al. (2025). Health Benefits of Palm Tocotrienol-Rich Fraction: A Scoping Review. Nutrition Reviews, 83(2):307–326.
https://academic.oup.com/nutritionreviews/article/83/2/307/7698337Pulliero A, et al. (2025). Antioxidant Food Supplementation in Cancer: Molecular Mechanisms and Clinical Evidence. International Journal of Molecular Sciences, 26(19):10234.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC12561932/Role of dietary antioxidants in diabetes: An overview (2024). Food Chemistry: X, 22:101350.
https://www.sciencedirect.com/science/article/pii/S2590157524000625
Disclaimer
This article is for educational purposes only. The information presented is based on scientific research but should not replace professional medical advice. Always consult with qualified healthcare providers before making changes to your health regimen, especially if you have existing medical conditions or are taking medications.
For more evidence-based health information and to explore Natural Health Connect's research-backed supplements, visit www.naturalhealthconnect.com.au
